Third Dose of Covid Vaccine for Children 5-11 Coming Soon
Pfizer will ask the FDA to grant emergency use authorization for its…
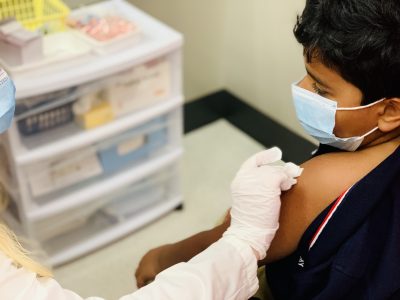
Pfizer will ask the FDA to grant emergency use authorization for its third dose of the COVID-19 vaccine for children between the ages of 5 and 11. According to the company, the third dose will help children to fend off the virus because it produced 36 times the protection against the Omicron strain when compared to the efficacy of the first two doses. The small population used for the study did not report any new adverse reactions. The children’s vaccine is different from the one made for people 12 and older, and the two should not be used interchangeably.

